Watch how ZIIHERA can induce immune-mediated cytotoxicity in multiple ways1
View transcript
ZIIHERA (zanidatamab-hrii) 50 mg/mL for Injection for IV is indicated for the treatment of adults with previously treated, unresectable or metastatic HER2-positive (IHC 3+) biliary tract cancer (BTC), as detected by an FDA-approved test. This indication is approved under accelerated approval based on overall response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
ZIIHERA has a BOXED Warning for embryo-fetal toxicity. Exposure to ZIIHERA during pregnancy can cause embryo-fetal harm. Advise patients of the risk and need for effective contraception.
Please see additional Important Safety Information at the end of this video.
The clinical relevance of the in vitro and in vivo data has not been established.
ZIIHERA is a dual HER2-targeted bispecific antibody with an asymmetrical design engineered to target HER2 at two distinct sites.
It is not a T-cell engager. Because ZIIHERA does not activate cytotoxic T cells, it has not been associated with cytokine release syndrome.
ZIIHERA is dual HER2-targeted, which means it is engineered to bind to two distinct sites on the HER2 receptor.
After this binding occurs, ZIIHERA may trigger tumor cell death through multiple mechanisms of action, including HER2 internalization, HER2 reduction, and activation of immune-mediated cytotoxicity.
Upon binding to HER2, the ZIIHERA-HER2 complex is internalized by the tumor cell. This leads to a reduction of HER2 on the tumor cell surface, which may inhibit tumor growth.
When on the cell surface, ZIIHERA may recruit the immune system to mediate the killing of tumor cells in three ways.
Immune-effector cells, like natural killer cells, may bind to ZIIHERA to initiate a process called antibody-dependent cellular cytotoxicity, or ADCC, while macrophages engulf cancer cells via antibody-dependent cellular phagocytosis, also known as ADCP.
At the same time, elsewhere on the tumor cell, complement proteins can be activated. Complement may bind to the ZIIHERA-HER2 complexes and ultimately kill the cancer cells through complement-dependent cytotoxicity, or CDC.
It is through all of these mechanisms of action that ZIIHERA may induce tumor cell death.
ZIIHERA is not a T-cell engager. It is a bispecific antibody with dual HER2-targeted binding properties. ZIIHERA binding to HER2 can result in:
- Internalization and HER2 reduction
- Induction of ADCC, ADCP, and CDC
- And targeted tumor cell death
Harness dual HER2 targeting with ZIIHERA for your patients with previously treated advanced HER2-positive (IHC 3+) biliary tract cancer.
WARNINGS AND PRECAUTIONS
Embryo-Fetal Toxicity
ZIIHERA can cause fetal harm when administered to a pregnant woman. In literature reports, use of a HER2-directed antibody during pregnancy resulted in cases of oligohydramnios and oligohydramnios sequence manifesting as pulmonary hypoplasia, skeletal abnormalities, and neonatal death.
Verify the pregnancy status of females of reproductive potential prior to the initiation of ZIIHERA. Advise pregnant women and females of reproductive potential that exposure to ZIIHERA during pregnancy or within 4 months prior to conception can result in fetal harm. Advise females of reproductive potential to use effective contraception during treatment with ZIIHERA and for 4 months following the last dose of ZIIHERA.
Left Ventricular Dysfunction
ZIIHERA can cause decreases in left ventricular ejection fraction (LVEF). LVEF declined by >10% and decreased to <50% in 4.3% of 233 patients. Left ventricular dysfunction (LVD) leading to permanent discontinuation of ZIIHERA was reported in 0.9% of patients. The median time to first occurrence of LVD was 5.6 months (range: 1.6 to 18.7). LVD resolved in 70% of patients.
Assess LVEF prior to initiation of ZIIHERA and at regular intervals during treatment. Withhold dose or permanently discontinue ZIIHERA based on severity of adverse reactions.
The safety of ZIIHERA has not been established in patients with a baseline ejection fraction that is below 50%.
Infusion-Related Reactions
ZIIHERA can cause infusion-related reactions (IRRs). An IRR was reported in 31% of 233 patients treated with ZIIHERA as a single agent in clinical studies, including Grade 3 (0.4%), and Grade 2 (25%). IRRs leading to permanent discontinuation of ZIIHERA were reported in 0.4% of patients. IRRs occurred on the first day of dosing in 28% of patients; 97% of IRRs resolved within one day.
Prior to each dose of ZIIHERA, administer premedications to prevent potential IRRs. Monitor patients for signs and symptoms of IRR during ZIIHERA administration and as clinically indicated after completion of infusion. Have medications and emergency equipment to treat IRRs available for immediate use.
If an IRR occurs, slow, or stop the infusion, and administer appropriate medical management. Monitor patients until complete resolution of signs and symptoms before resuming. Permanently discontinue ZIIHERA in patients with recurrent severe or life-threatening IRRs.
Diarrhea
ZIIHERA can cause severe diarrhea.
Diarrhea was reported in 48% of 233 patients treated in clinical studies, including Grade 3 (6%) and Grade 2 (17%). If diarrhea occurs, administer antidiarrheal treatment as clinically indicated. Perform diagnostic tests as clinically indicated to exclude other causes of diarrhea. Withhold or permanently discontinue ZIIHERA based on severity.
ADVERSE REACTIONS
Serious adverse reactions occurred in 53% of 80 patients with unresectable or metastatic HER2-positive BTC who received ZIIHERA. Serious adverse reactions in >2% of patients included biliary obstruction (15%), biliary tract infection (8%), sepsis (8%), pneumonia (5%), diarrhea (3.8%), gastric obstruction (3.8%), and fatigue (2.5%). A fatal adverse reaction of hepatic failure occurred in one patient who received ZIIHERA.
The most common adverse reactions in 80 patients with unresectable or metastatic HER2-positive BTC who received ZIIHERA (≥20%) were diarrhea (50%), infusion-related reaction (35%), abdominal pain (29%), and fatigue (24%).
USE IN SPECIFIC POPULATIONS
Pediatric Use
Safety and efficacy of ZIIHERA have not been established in pediatric patients.
Geriatric Use
Of the 80 patients who received ZIIHERA for unresectable or metastatic HER2-positive BTC, there were 39 (49%) patients 65 years of age and older. Thirty-seven (46%) were aged 65-74 years old and 2 (3%) were aged 75 years or older.
No overall differences in safety or efficacy were observed between these patients and younger adult patients.
Please see full Prescribing Information, including BOXED Warning, at ZIIHERAhcp.com for further information.
ZIIHERA binds to 2 distinct sites on HER21,2
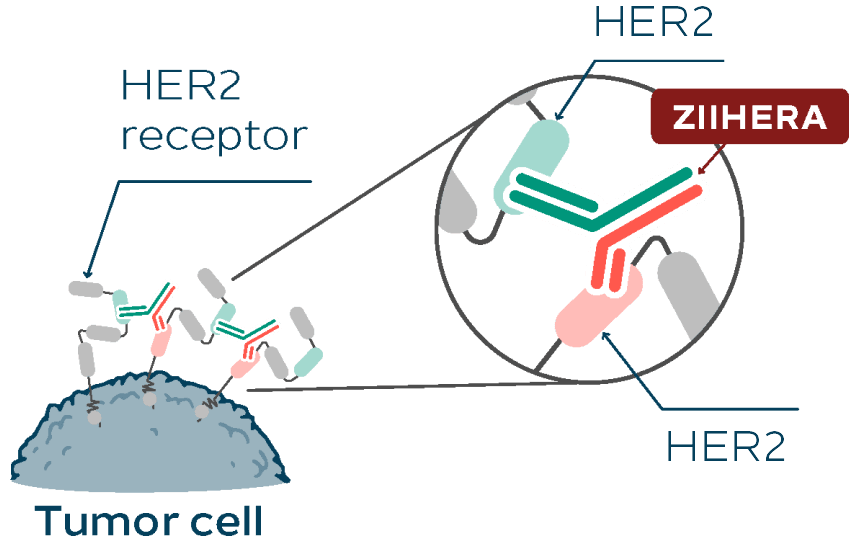
ZIIHERA is not a T-cell engager1 This representation is one way ZIIHERA may bind to HER2.
ZIIHERA may lead to tumor cell death through multiple mechanisms of action (MOA)*:
HER2 internalization1
Reduction of HER2 on cell surface1
Induction of immune-mediated cytotoxicity (CDC, ADCC, and ADCP)1
TUMOR CELL DEATH

ZIIHERA IS ENGINEERED TO INHIBIT TUMOR GROWTH WITH BISPECIFIC HER2 TARGETING1
*These mechanisms result in tumor growth inhibition cell death in vitro and in vivo.1
ADCC=antibody-dependent cellular cytotoxicity; ADCP=antibody-dependent cellular phagocytosis; CDC=complement-dependent cytotoxicity; FDA=Food and Drug Administration; HER2=human epidermal growth factor receptor 2; IHC=immunohistochemistry.